Sort the components into the correct solubility. – Sorting the components into the correct solubility is a fundamental aspect of chemistry that plays a vital role in numerous scientific disciplines and everyday applications. This guide delves into the intricacies of solubility, exploring the factors that influence it and providing a practical approach to sorting components based on their solubility.
By understanding the principles of solubility, scientists and researchers can optimize chemical reactions, develop innovative drug formulations, and enhance the quality of food products. In this comprehensive guide, we will unravel the mysteries of solubility, empowering readers with the knowledge and techniques to effectively sort components and harness their unique properties.
Solubility Definitions

Solubility refers to the ability of a substance (solute) to dissolve in another substance (solvent), forming a homogeneous mixture called a solution. The extent of solubility is expressed in terms of concentration, which can be measured in various units such as molarity, molality, or mass percentage.
Different substances exhibit varying degrees of solubility. For instance, sugar is highly soluble in water, whereas sand is practically insoluble. Factors like temperature, pressure, and the nature of the solute and solvent influence solubility.
Components of a Solution: Sort The Components Into The Correct Solubility.
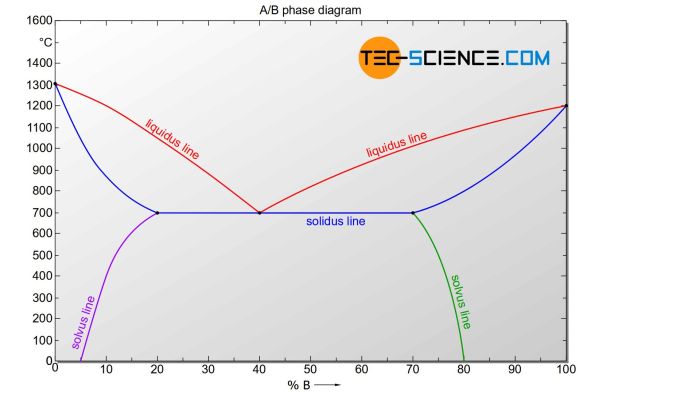
A solution comprises two main components: solute and solvent. The solute is the substance being dissolved, while the solvent is the substance in which the solute dissolves. The concentration of a solution indicates the amount of solute present in a given amount of solvent.
Solutions can be classified into different types based on their concentration. Saturated solutions contain the maximum amount of solute that can dissolve at a given temperature and pressure. Unsaturated solutions have less solute than a saturated solution, while supersaturated solutions contain more solute than a saturated solution at a given temperature and pressure.
Sorting Components by Solubility
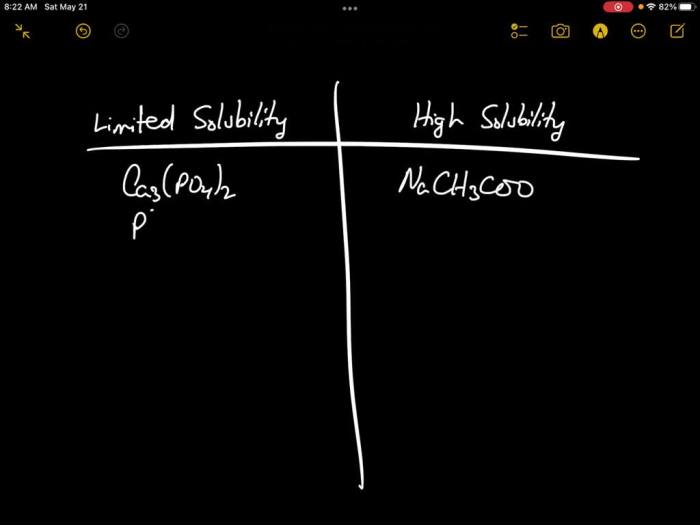
To sort components based on their solubility, an experiment can be designed involving the following steps:
- Select a variety of components with unknown solubilities.
- Prepare a set of solvents with varying polarities (e.g., water, ethanol, hexane).
- Add a small amount of each component to each solvent and observe whether it dissolves or not.
- Record the results in a table, indicating the solubility of each component in each solvent.
By analyzing the results, patterns or trends in the solubility of different components can be identified. For example, components with similar chemical structures or polarities tend to have similar solubilities.
Applications of Solubility
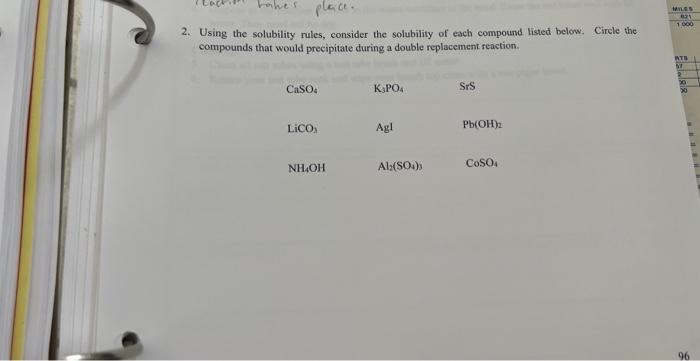
Solubility has numerous practical applications in various fields:
- Chemistry:Solubility plays a crucial role in chemical reactions, purification processes, and the development of new materials.
- Medicine:The solubility of drugs determines their bioavailability, effectiveness, and side effects.
- Food industry:Solubility is essential in food processing, preservation, and the development of new food products.
In everyday life, solubility is encountered in various situations, such as dissolving sugar in tea, extracting flavors from herbs, or the formation of clouds and precipitation.
Frequently Asked Questions
What is the definition of solubility?
Solubility refers to the ability of a substance (solute) to dissolve in a solvent to form a homogeneous mixture known as a solution.
What factors affect the solubility of a substance?
Temperature, pressure, polarity, and the nature of the solute and solvent all influence the solubility of a substance.
How can we sort components based on their solubility?
By designing experiments that involve dissolving components in different solvents and observing their solubility behavior, we can sort components based on their solubility.
